
As effective as homeopathy?
Earlier this year I noted that the FDA had committed to re-evaluating a popular but ineffective cold remedy ingredient, phenylephrine. The Non-prescription Drug Advisory Committee met in September 2023 to discuss the effectiveness of phenylephrine as an ingredient – both as a single agent (like the box above) and when combined with other ingredients. Not surprisingly, and consistent with the accumulated data, the committee concluded that the evidence does not support that phenylephrine is effective as a nasal decongestant.
What is phenylephrine?
In 1976, the FDA concluded that three medicines were safe and effective for the treatment of nasal congestion caused by colds, allergies, and sinusitis: phenylpropanolamine, pseudoephedrine, and phenylephrine. Phenylpropanolamine was subsequently withdrawn from the market after use was associated with strokes. Pseudoephedrine is an effective decongestant. However it is chemically very similar to methamphetamine, an addictive and controlled drug that subject to abuse. Because pseudoephedrine can be used to manufacture methamphetamine, the over-the-counter sale of cough and cold products that contain this ingredient are not permitted in the USA. Sales were moved “behind the counter”, with the requirement for the customer to produce photo identification and the requirement for the pharmacy to maintain a log of all sales, tracked by customer name and address. (This restriction does not exist in other countries.)
When pseudoephedrine was moved behind the counter, manufacturers of cough and cold products either had to accept a much smaller market for their products, or find a substitute. Some companies reformulated their products to contain phenylephrine instead, the last remaining non-prescription ingredient that the FDA had approved for congestion. Phenylephrine is from a category of drugs called alpha-1 agonists, that stimulate alpha-1 receptors that are present throughout the body, including the nasal passages. It is used as a nasal spray, but more commonly as a oral product take to treat nasal congestion. There was a problem with FDA’s approval, however. There was little convincing evidence phenylephrine, when taken orally, was effective.
It may not be effective. But you cannot make methamphetamine from it.
The evidence against phenylephrine
Phenylephrine is a poorly-absorbed drug (i.e., it has low bioavailability). Most of the drug is broken down during the absorption process and after passing through the liver. Only an estimated 38% of a dose actually reaches the bloodstream (compared with 90% for pseudoephedrine). In clinical trials, phenylephrine has been compared to other decongestants and has not been shown to be effective. The fact that we are relying on trials conducted in the 1970’s pretty much tells us what we need to know about phenylephrine. Given how poorly it is absorbed, there is the question of whether simply giving bigger doses will work – and it turns out it may. However, the 10mg dose is what’s available in US cough and cold products – and the best evidence shows that this drug is effectively a placebo at that dose. In a review of the data that the FDA had considered, that review noted that for the 10mg dose, 4 studies showed efficacy and seven showed no difference from placebo. The authors concluded that a systematic review did not support the FDA’s decision. Other trials conducted since that time (like this one in 2009) are supportive of the conclusion that the 10mg dose is ineffective.
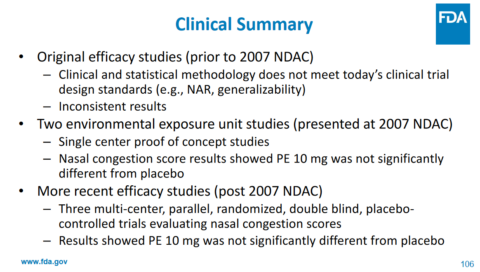
In September 2023, the FDA reviewed the evidence to support phenylephrine as a cough and cold ingredient. Here are a few of the key slides and conclusions:

Phenylephrine has action against alpha receptors – not its metabolites. Less than 1% of the drug is actually absorbed as the “active” drug. The maximum effective blood concentration (Cmax) is lower than the EC50, which is half maximal effective concentration. The drug is quickly eliminated from the body. And here is their bottom line on the clinical data, which won’t be surprising based on the pharmacology of the drug in the body:

The agency commented that the data available today refutes some of the assumptions made back in the 1970’s (pg. 55):
Finally, the science has changed in the interim, and a full understanding of the clinical pharmacology of PE [phenylephrine] when administered orally was not appreciated at the time that the Panel issued its recommendations. We now know that in addition to PE having less than 1% systemic bioavailability, the half-life is also significantly shorter than the original 4-hour dosing interval. These data were not available to the Panel, nor was the technology to fully assess oral bioavailability available until around the turn of this century. Therefore, is it not surprising that the Panel considered the equivocal findings in the original studies as sufficient to support a finding of efficacy (and safety) of PE 10 mg administered orally every 4 hours.
and, interestingly (pg. 60),
After careful review, we note that there were many methodological and statistical issues with these studies. We believe that these issues significantly limit any judgement of a statistical “win”, and more importantly a clinical “win”, for any of these studies. In fact, if these studies were submitted to the Agency today all would at most be considered Phase 1 studies at best. As a result, we believe that these studies do not stand up to the new data that are now available with regard to the efficacy of orally administered PE as a nasal decongestant.
Ultimately, the committee unanimously concluded that the evidence does not support that the recommended dosage of orally administered phenylephrine is effective as a nasal decongestant. No safety issues with use of oral phenylephrine at the recommended dose were identified.
Is this the end of phenylephrine?
The advisory committee’s conclusion was consistent with what many cold sufferers already knew: This drug doesn’t work.
The FDA’s briefing points out important benefits of changing the status of phenylephrine: reducing health care costs from taking a drug with no benefit, reducing the risk of allergic reactions or other side effects (when used in combination with other products), avoiding risk (especially with combination products) of taking an excessive amount in order to seek some benefit, minimizing risk to children, and importantly, it eliminates the missed opportunity to use a product that actually works.
This isn’t a final decision – yet. A advisory committee provides independent advice and recommendations to FDA. It will consider the input of this advisory committee before making a decision about the ongoing availability of oral phenylephrine. With this ingredient in which $1.8 billion of cough and cold products sold in the USA, it will be interesting to see how manufacturers (and consumers) of these products will react, given the advisory committee’s advice.

